ABSTRACT:
The residue-on-evaporation method of purified water is to detect the quality of residue on evaporation solids obtained after 100 mL of purified water is evaporated and dried. The difficulty is that the operator needs to repeat the operation to achieve the 0.3mg constant weight value required by the Chinese Pharmacopoeia. The traditional test method has the shortcomings of low efficiency and manual duty. After long-term practice and repeated verification, it adopts an integrated automatic structure design, equipped with rapid water bath evaporation and gas balance technology, which can realize automatic feeding, water bath evaporation, blast drying and cooling balance, constant temperature weighing five-in-one automatic test. Through the repeatability experiment and gradient design experiment, the results show that: 2 rounds of weighing can reach a constant weight error value of 0.3mg, when the scalar amount is 9.4mg/100mL, the precision (RSD) is 0.73%, and the accuracy of the gradient design experiment is high. There is no deviation between parallel samples. Conclusion: After the innovation of the residue on evaporation detection technology of purified water, the test time to reach constant mass value is short, the operation is simple, and the data accuracy and precision are good.
KEYWORDS: purified water; residue on evaporation; constantmass; automated test
01
Standard requirements
According to the requirements of Chinese Pharmacopoeia 2020 Edition Part II "Purified Water": Take 100mL of this product, put it in an evaporating dish with constant weight at 105°C, evaporate it to dryness on a water bath, and dry it at 105°C to constant weight, and the remaining residue should not exceed 1mg. The Chinese Pharmacopoeia defines constant weight as constant weight, unless otherwise specified, refers to the weight of the test sample after two consecutive drying or igniting weighing the difference is less than 0.3mg; the second drying to constant weight and each subsequent weighing should be carried out after 1 hour of drying under the specified conditions.
According to the requirements of European Pharmacopoeia 10.0 Edition, about “Purified water in containers“,
Residue on evaporation: maximum 0.001 per cent. Evaporate 100 mL to dryness on a water-bath and dry in an oven at 100-105 °C. The residue weighs a maximum of 1 mg.
The requirements of the Chinese Pharmacopoeia, the US Pharmacopoeia and the European Pharmacopoeia for limits of constant mass and residue on evaporation in purified water are shown in Table 1. It is worth noting that the US Pharmacopoeia does not require residue-on-evaporation items for purified water, the European Pharmacopoeia has no residue on evaporation testing items for Purified water in bulk, while Purified water in containers does not. For residue on evaporation testing items, the limit requirement is also ≤10mg/L, but there is no requirement for constant weight. Bulk purified water usually refers to the water flowing in the pipeline, which is commonly referred to as "living water", while purified water is stored in containers, commonly known as "dead water"; relatively speaking, the requirement of purified water in containers is higher.

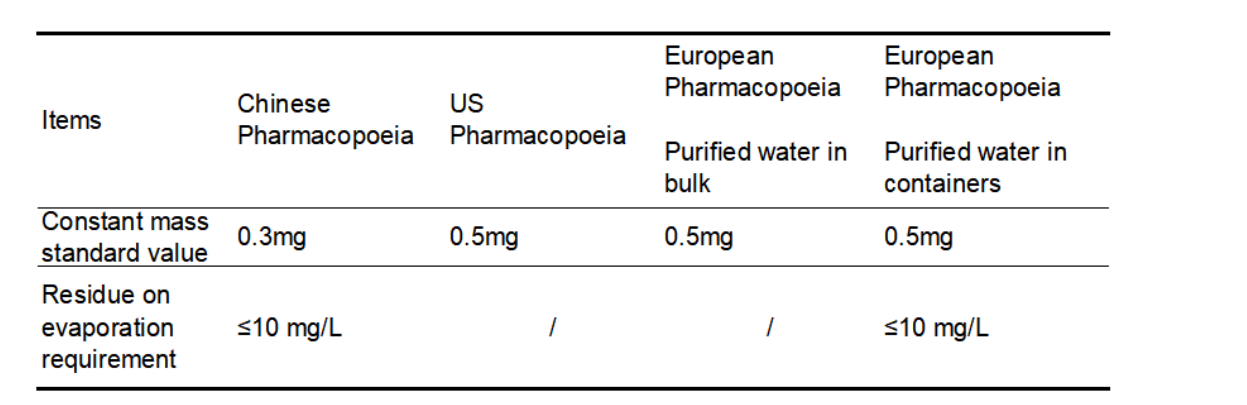
In general, the requirements for purified water in the Chinese Pharmacopoeia are more stringent than those in the European and American Pharmacopoeia.
02
Detection technology innovation
In view of the difficulty of analyzing and testing personnel operating the residue on evaporation in purified water project, after a lot of test verification and experience summary, the project has carried out technical innovation, and the specific performance is as follows:
01/
A fully automatic total migration and residue on evaporation tester integrating functions such as automatic feeding, water bath evaporation, heating and drying, drying and cooling, and constant temperature weighing. The integrated control system of cold and hot dual chambers, all operations are carried out in the same closed chamber, which isolates the influence of external factors on the test, and the test is efficient and convenient to automate. Break through the traditional manual testing and realize fully automated testing.
02/
In order to meet the high-throughput and high-precision test requirements, 36 test stations are designed, and 3 high-precision imported balances (resolution 0.01mg) are configured, which is higher than the standard required balance accuracy.
03/
The core component weighing template (balance) completely isolates the test chamber, and is placed at the lower end of the instrument with a stable structure. The balance is placed in a thermal insulation cover connected to circulating water. The equipment balance module is also designed with an isolation device. When performing weighing steps, isolation The device is only turned on for weighing, and turned off during steps such as water bath evaporation, heating and drying.
04/
The software displays weighing data in real time, and has functions such as audit tracking and authority management, which can meet the needs of the pharmaceutical industry for data traceability.
03
Experiment
3.1 Materials and Instruments
Distilled water (grade 1), Bonfei;
Sodium chloride (Analytical Reagent, AR), Xilong Science;
Purified water (added sodium chloride), sample 1~sample 5, provided by the customer;
Analytical balance (divided 0.1mg), AR224CN, OHRUS;
AUTO ZF3600 Full-automatic overall Migration Tester , Guangzhou Biaoji Packaging Equipment Co., Ltd.

3.2 Test process
Sample preparation: Weigh 0.0940g of sodium chloride into a volumetric flask containing 1000mL of distilled water, and shake well. Measure 100 mL with a graduated cylinder and place them in the automatic feeding solvent of the AUTO ZF3600 tester, take 7 parallel samples, and make 3 blank solutions at the same time. A total of 10 solutions to be tested. In addition, measure 100 mL of sample 1 with a graduated cylinder and add it to the solvent holding dish, and take 3 parallel samples for each sample. A total of 15 solutions to be tested.
Parameter setting: empty cup heating time 60min, empty cup cooling time 60min, water bath evaporation time 300min, residue constant weight heating time 60min, residue constant weight cooling time 60min, cavity heating temperature 105℃, cavity cooling temperature 29℃, water bath temperature 100°C. Feeding method: automatic (non-pause), constant weight standard value 0.30mg.

3.3 Results and Discussion
By adding a known amount of sodium chloride as a residue on evaporation to purified water, and simulating the residue on evaporation test of purified water with known results, the data accuracy and precision of the AUTO ZF3600 tester are verified. Empty cups were weighed at constant weight, and residues were weighed in 2 rounds. The difference between the constant weights was less than 0.3 mg. The repeatability test results of sodium chloride solution are shown in Table 2. The experimental results of the sodium chloride solution gradient design are shown in Table 3.
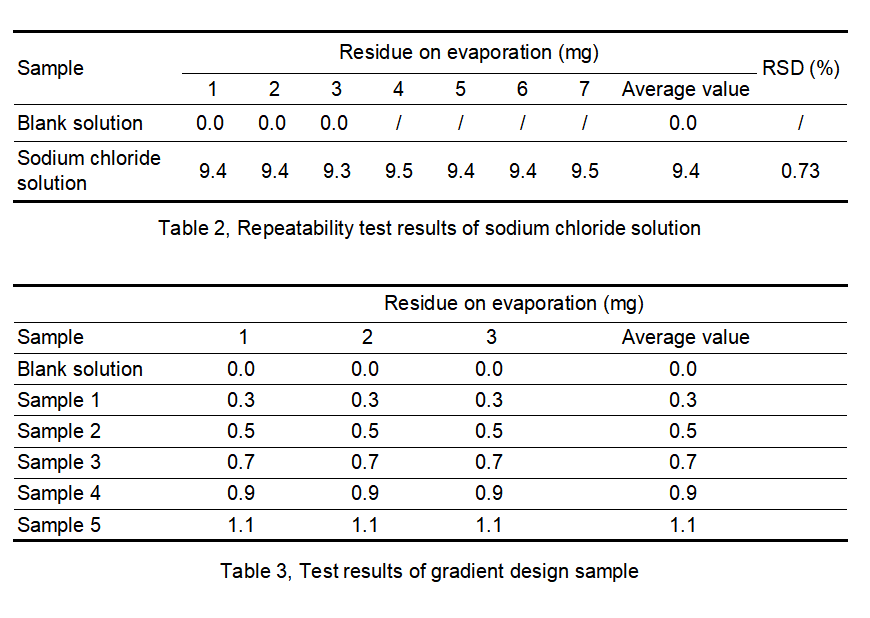
From the data analysis in Table 2, it can be seen that the designed addition amount is 9.4 mg/100 mL, the measured residue on evaporation is 9.4 mg, and the RSD is 0.73%. The AUTO ZF3600 tester has good precision in the repeatability test.
From the data analysis in Table 3, it can be seen that the value of the low addition amount (0.3 mg) and the value near the limit specified by the pharmacopoeia (0.9 mg, 1.1 mg) can be accurately weighed, and the accuracy of the results is good, and there is no deviation between parallel samples.
04
Conclusion

The residue on evaporation in purified water is one of the basic must-check items in the pharmaceutical industry, and its quality directly affects the quality of medicines. Using traditional test methods, it is difficult to meet the standard value of 0.3mg constant weight specified by the Chinese Pharmacopoeia. At this stage, intelligent instruments adopt integrated technological innovation, which can easily realize automated and efficient testing. In this paper, by adding a known amount of sodium chloride as a residue on evaporation, the repeatability and gradient design experiments are carried out on the purified water sample. The results show that the test results have good accuracy after using intelligent equipment for the residue on evaporation in purified water project. At the same time, it solves the industry problems such as the difficulty of constant weight, long time consumption, and untraceable data of the residue on evaporation in purified water project.

We GBPI is a professional manufacturer focused on developing and producing packaging material test instruments. Committed to providing comprehensive, professional and high-quality products and technical services for packaging, food, medicine, testing and other industries. Founded in 2002, the company has received IOS9001 certification and recognized as high-tech enterprise and software enterprise by national authority. We hold numerous technology patents and software certificates from the national authority, and gained awards for technological progress.

 info@gbtest.cn
info@gbtest.cn



 en
en ru
ru es
es ar
ar





