Summary
The non-volatile matter test principle of purified water is to detect the mass of non-volatile matter solids obtained after 100mL of purified water is evaporated and dried. The difficulty is that testers need to perform repeated operations to achieve the 0.3mg constant weight value required by the Chinese Pharmacopoeia. The traditional test method has the disadvantages of low efficiency and manual on-duty. After long-term practice and repeated verification, it adopts an integrated and automated structural design, equipped with rapid water bath evaporation and gas balance technology, which can realize automatic feeding, water bath evaporation, blast drying, and cooling. Balance, constant temperature and weighing five in one fully automatic test. Through repeated experiments and gradient design experiments, the results show that: 2 rounds of weighing can reach a constant weight error of 0.3mg, and when the amount of scalar addition is 9.4mg/100mL, the precision (RSD) is 0.73%, and the accuracy of the gradient design experiment is high. There is no bias between parallel samples. Conclusion: After the innovation of the non-volatile detection technology of purified water, the constant weight time is short, the operation is simple, and the data accuracy and precision are good.
Key words: purified water; non-volatile matter; constant weight; automated testing

In the production of pharmaceuticals, purified water is widely used as solvents, detergents, auxiliary materials, raw materials for pure steam and water for injection, etc., from raw materials to preparations, the use of purified water runs through the entire production process. Therefore, the quality control of purified water is very important in the production management of pharmaceutical companies. The test of non-volatile matter in purified water is one of the basic inspection items. The difficulty lies in the 0.3mg constant weight value required by the Chinese Pharmacopoeia. Experimental stop condition. Traditional testing methods have disadvantages such as low efficiency, repetitive and cumbersome operations, difficulty in constant weight, and the need for manual supervision.
Domestic and foreign standard requirements
According to the requirements of "Purified Water" in the second part of the Chinese Pharmacopoeia 2020 edition: take 100mL of this product, put it in an evaporating dish with a constant weight at 105°C, evaporate it to dryness on a water bath, and dry it to a constant weight at 105°C. The remaining residue should not exceed 1mg. The definition of constant weight in the Chinese Pharmacopoeia is constant weight, unless otherwise specified, refers to the weight of the test sample whose weight difference is less than 0.3 mg after two consecutive dryings or igniting; the second time of drying to constant weight And every subsequent weighing should be carried out after continuing to dry for 1 hour under the specified conditions.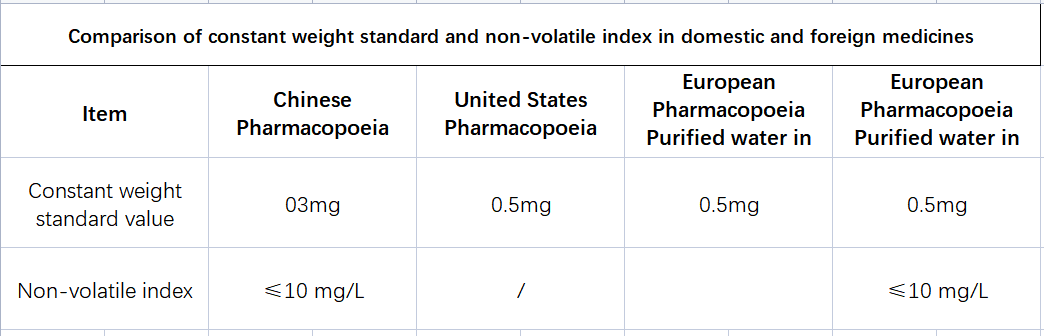
See Table 1 for the requirements of the Chinese Pharmacopoeia, the United States Pharmacopoeia and the European Pharmacopoeia for constant mass and non-volatile matter limits in purified water. It is worth noting that the United States Pharmacopoeia has no requirements for the non-volatile items of purified water, while the bulk purified water (Purified water in bulk) in the European Pharmacopoeia has no non-volatile items, while the barrel-packed purified water (Purified water containers) has no requirements. For the volatile matter detection items, the limit value requirement is also ≤10mg/L, but there is no requirement for constant weight. Bulk purified water usually refers to the water flowing in the pipeline, which is commonly referred to as "living water", while barreled purified water is packed in barrels, commonly known as "dead water"; relatively speaking, the requirements for barreled purified water higher.
Generally speaking, compared with the European and American Pharmacopoeia, the Chinese Pharmacopoeia's requirements for purified water items are more stringent in terms of indicators.
Detection technology innovation
Aiming at the difficulties of analysts and testers in operating the purified water non-volatile project, after a large number of test verifications and experience summarization, a technical innovation has been carried out for this project, the specific performance is as follows:
01/
It is a fully automatic total migration and non-volatile matter tester that integrates functions such as automatic feeding, water bath evaporation, heating and drying, drying and cooling, and constant temperature weighing. The cold and hot dual chamber integrated control system, all operations are carried out in the same closed chamber, which isolates the influence of external factors on the test, and the test is efficient, convenient and automated. Break through traditional manual testing and realize fully automated testing.
02/
In order to meet the high-throughput and high-precision test requirements, 36 test stations are designed and equipped with 3 high-precision imported balances (resolution 0.01mg), which are higher than the balance precision required by the standard.
03/
The weighing template (balance) of the core component is completely isolated from the test chamber. It is placed at the lower end of the instrument with a stable structure. The balance is placed in a heat preservation cover connected to circulating water. The balance module of the equipment is also designed with an isolation device. The device is opened for weighing, and it is closed during steps such as water bath evaporation, heating and drying.
04/
The software displays weighing data in real time, and has functions such as audit trail and authority management, which can meet the needs of the pharmaceutical industry for data traceability.
Experiment
3.1 Materials and Instruments
Distilled water (grade 1), Bonfilis;
Sodium chloride (analytical pure), Xilong Science;
Purified water (with sodium chloride added), sample 1~sample 5, provided by the customer;
Analytical balance (graduation 0.1mg), AR224CN, OHRUS;
AUTO ZF3600 total migration and non-volatile tester, GBPI

3.2 Test process
Sample preparation: Weigh 0.0940g of sodium chloride into a volumetric flask filled with 1000mL of distilled water, and shake well. Use a graduated cylinder to measure 100mL to the automatic feeding solvent placement dish of the AUTO ZF3600 total migration and non-volatile tester, take 7 parallel samples, and make 3 blank solutions at the same time. A total of 10 solutions to be tested. In addition, measure 100 mL of sample 1 with a graduated cylinder and add it to the solvent storage dish, and take 3 parallel samples for each sample. A total of 15 solutions to be tested.
Parameter setting: empty cup heating time 60min, empty cup cooling time 60min, water bath evaporation time 300min, residue constant weight heating time 60min, residue constant weight cooling time 60min, cavity heating temperature 105°C, cavity cooling temperature 29°C, water bath temperature 100°C. Feeding method: automatic (non-pause), constant weight standard value 0.30mg.
Parameter setting: empty cup heating time 60min, empty cup cooling time 60min, water bath evaporation time 300min, residue constant weight heating time 60min, residue constant weight cooling time 60min, cavity heating temperature 105°C, cavity cooling temperature 29°C, water bath temperature 100°C. Feeding method: automatic (non-pause), constant weight standard value 0.30mg.

3.3 Results and discussion
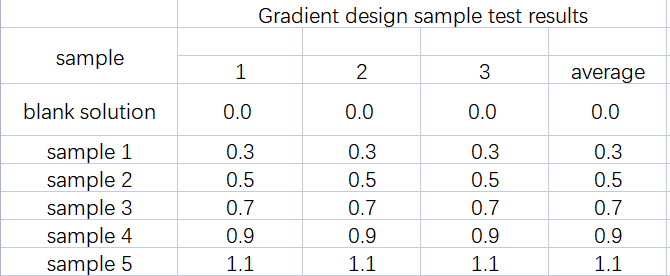
By adding a known amount of sodium chloride as non-volatile matter to purified water, and simulating the non-volatile matter test of purified water with known results, the data accuracy and precision of the AUTO ZF3600 total migration and non-volatile tester are verified. The constant weight of the empty cup and the constant weight of the residue were completed in 2 rounds, and the difference between the constant weights was less than 0.3mg. The repeatability test results of sodium chloride solution are shown in Table 2. Table 3 shows the experimental results of the sodium chloride solution gradient design.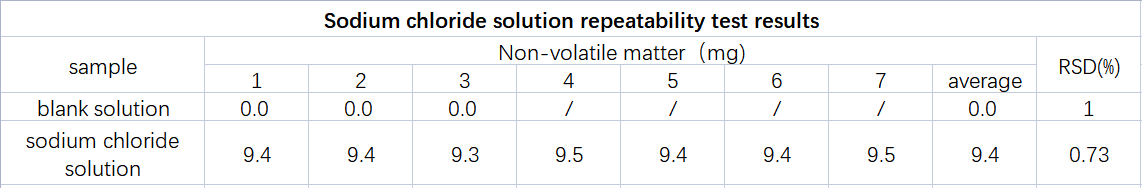
From the data analysis in Table 2, it can be seen that the designed addition amount is 9.4mg/100mL, the measured non-volatile matter is 9.4mg, and the RSD is 0.73%. The AUTO ZF3600 total migration and non-volatile measuring instrument has good precision in the repeatability test .
From the analysis of the data in Table 3, it can be seen that the value of the lower added amount (0.3 mg) and the value near the limit of the Pharmacopoeia (0.9 mg, 1.1 mg) can be accurately weighed, the accuracy of the results is good, and there is no deviation between parallel samples .
The non-volatile matter item in purified water is one of the basic inspection items in the pharmaceutical industry, and its quality directly affects the quality of drugs. Using traditional testing methods, it is difficult to meet the standard value of 0.3 mg constant weight stipulated in the Chinese Pharmacopoeia. At this stage, intelligent instruments adopt integrated technological innovation, which can easily realize automated and efficient testing. In this paper, through the study of adding a known amount of sodium chloride as a non-volatile matter, the repeatability and gradient design experiments were carried out on purified water samples. The results showed that after using intelligent equipment for the purified water non-volatile matter project, the test results have good accuracy At the same time, it solves the industry problems such as difficult constant weight, long time-consuming, and untraceable data in the purified water non-volatile matter project.

 info@gbtest.cn
info@gbtest.cn



 en
en ru
ru es
es ar
ar





